Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
CLINICAL RESEARCH
A comprehensive analysis of immune characteristics and clinical prognosis in Asian COVID-19 patients infected with SARS-CoV-2 Omicron strain XBB sub-variants: a retrospective study of 450 cases
1
Core Laboratory, Tianjin Beichen Hospital, Tianjin, China
2
Department of Oncology, Tianjin Beichen Hospital, Tianjin, China
3
Department of Respiratory and Critical Care, Tianjin Beichen Hospital, Tianjin, China
These authors had equal contribution to this work
Submission date: 2023-10-05
Final revision date: 2024-01-08
Acceptance date: 2024-01-08
Online publication date: 2024-12-13
KEYWORDS
TOPICS
ABSTRACT
Introduction:
The pandemic of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has posed a severe threat to human health since December 2019. Immune characteristics and clinical symptoms manifested by COVID-19 patients of the most recent new strains have not been reported.
Material and methods:
We retrospectively investigated 450 patients with laboratory-confirmed COVID-19 infection from December 2022 to January 2023. Clinical information and peripheral blood of the patients were obtained and analyzed for serum IL-6 levels and T cell sub-types. Post hoc analysis was performed to uncover immunological and involved COVID-19-associated pneumonia differences between patients with different underlying diseases and ages.
Results:
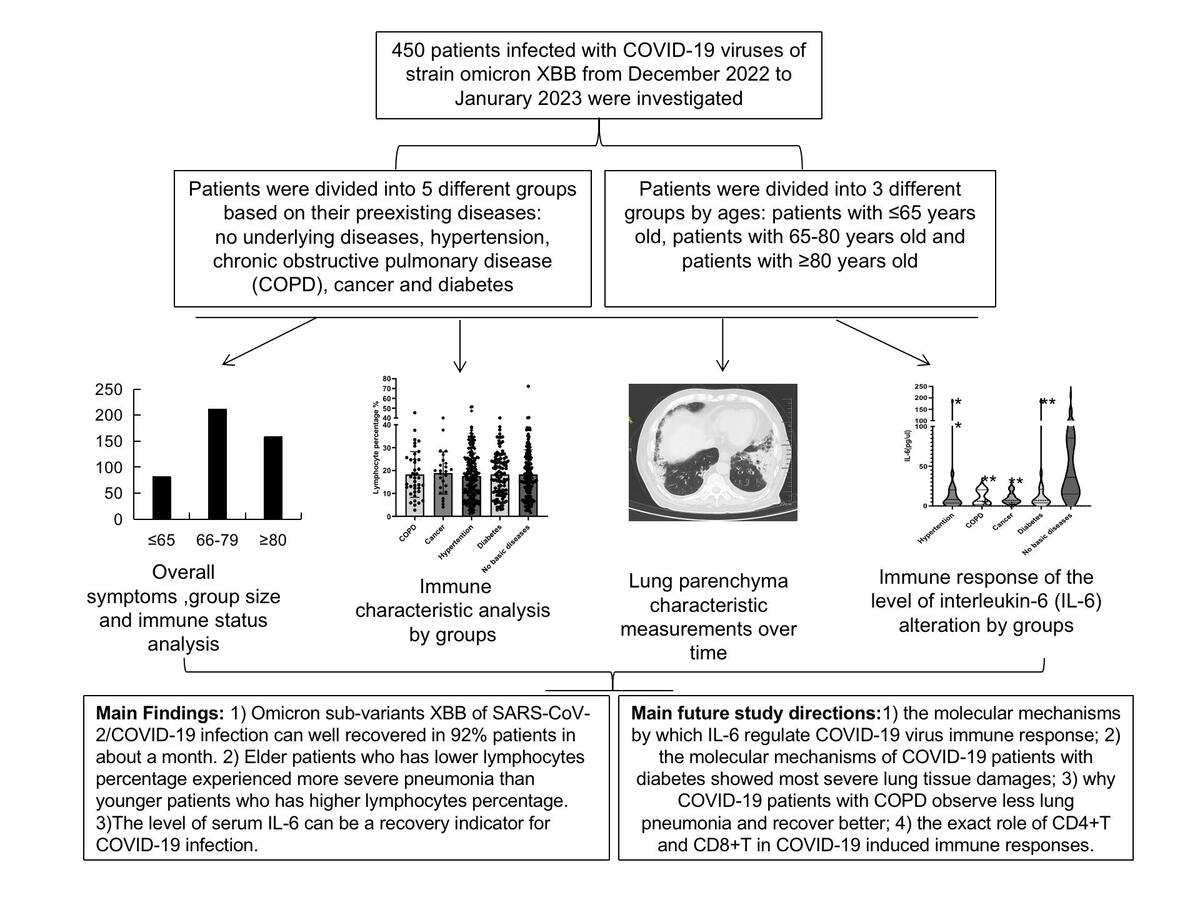
The median age of the patients was 75.5 years old. 60% of the patients were male and 40% were female. The most common symptoms were cough (344/450,76.4%), fever (317/450, 70.4%), expectoration (199/450, 44.2%) and wheeze (143/450, 31.8%). The mean hospital stay was 11.85 days (range: 1–57). 92% of the patients recovered in a month. The level of serum IL-6 was significantly higher in patients without underlying diseases compared with patients with hypertension, chronic obstructive pulmonary disease (COPD), cancer and diabetes (p < 0.001). Serum IL-6 level was significantly higher in patients who were 66–79 years old than that in patients aged 65 years and younger (p < 0.001). Peripheral CD8+T cell percentage was significantly higher in patients aged 65 years and younger than that in patients aged 80 years and older (p = 0.05). The mean involved ground-glass opacity area of the lung of all studied patients found by chest computed tomography (CT) at the time of initial onset of symptoms was 35.7%. Fifty-seven out of 132 (43.2%) patients who had assessable CT scans at 4–12 weeks after infection completely recovered with no chest CT abnormality. Involved ground-glass area of the lung of patients with diabetes or without underlying disease was significantly more severe than that in patients with COPD (p = 0.041 and p = 0.017, respectively). Involved ground-glass area of the lung of patients aged 80 years and older was significantly more severe than that in patients aged 65 years and younger (p = 0.031).
Conclusions:
92% of COVID-19 patients infected with Omicron XBB sub-variants of SARS-CoV-2 can recover well in a month. Patients aged 80 years and older who have a lower lymphocyte percentage experienced more severe pneumonia than patients aged 65 years and younger having a higher lymphocyte percentage. Serum IL-6 level can be a recovery indicator for patients with COVID-19 infection.
The pandemic of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has posed a severe threat to human health since December 2019. Immune characteristics and clinical symptoms manifested by COVID-19 patients of the most recent new strains have not been reported.
Material and methods:
We retrospectively investigated 450 patients with laboratory-confirmed COVID-19 infection from December 2022 to January 2023. Clinical information and peripheral blood of the patients were obtained and analyzed for serum IL-6 levels and T cell sub-types. Post hoc analysis was performed to uncover immunological and involved COVID-19-associated pneumonia differences between patients with different underlying diseases and ages.
Results:
The median age of the patients was 75.5 years old. 60% of the patients were male and 40% were female. The most common symptoms were cough (344/450,76.4%), fever (317/450, 70.4%), expectoration (199/450, 44.2%) and wheeze (143/450, 31.8%). The mean hospital stay was 11.85 days (range: 1–57). 92% of the patients recovered in a month. The level of serum IL-6 was significantly higher in patients without underlying diseases compared with patients with hypertension, chronic obstructive pulmonary disease (COPD), cancer and diabetes (p < 0.001). Serum IL-6 level was significantly higher in patients who were 66–79 years old than that in patients aged 65 years and younger (p < 0.001). Peripheral CD8+T cell percentage was significantly higher in patients aged 65 years and younger than that in patients aged 80 years and older (p = 0.05). The mean involved ground-glass opacity area of the lung of all studied patients found by chest computed tomography (CT) at the time of initial onset of symptoms was 35.7%. Fifty-seven out of 132 (43.2%) patients who had assessable CT scans at 4–12 weeks after infection completely recovered with no chest CT abnormality. Involved ground-glass area of the lung of patients with diabetes or without underlying disease was significantly more severe than that in patients with COPD (p = 0.041 and p = 0.017, respectively). Involved ground-glass area of the lung of patients aged 80 years and older was significantly more severe than that in patients aged 65 years and younger (p = 0.031).
Conclusions:
92% of COVID-19 patients infected with Omicron XBB sub-variants of SARS-CoV-2 can recover well in a month. Patients aged 80 years and older who have a lower lymphocyte percentage experienced more severe pneumonia than patients aged 65 years and younger having a higher lymphocyte percentage. Serum IL-6 level can be a recovery indicator for patients with COVID-19 infection.
REFERENCES (30)
1.
Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708-20.
2.
Chiranjib C, Manojit B, Kuldeep D. SARS-CoV-2 vaccines, vaccine development technologies, and significant efforts in vaccine development during the pandemic: the lessons learned might help to Fight against the next pandemic. Vaccines (Basel) 2023; 11: 682.
3.
Bhattacharjee A, Saha M, Halder A, Debnath A, Mukherjee O. Therapeutics and vaccines: strengthening our fight against the global pandemic COVID-19. Curr Microbiol 2021; 78: 435-48.
4.
Bahrami A, Azargoonjahromi A, Sadraei S, et al. An overview of current drugs and prophylactic vaccines for coronavirus disease 2019 (COVID-19). Cell Mol Biol Lett 2022; 27: 38.
5.
Toubasi AA, Al-Sayegh TN, Obaid YY, et al. Efficacy and safety of COVID-19 vaccines: a network meta-analysis. J Evid Based Med 2022; 15: 245-62.
6.
Abulsoud AI, El-Husseiny HM, El-Husseiny AA, et al. Mutations in SARS-CoV-2: insights on structure, variants, vaccines, and biomedical interventions. Biomed Pharmacother 2023; 157: 113977.
7.
Almalki OS, Santali EY, Alhothali AA, et al. The role of blood groups, vaccine type and gender in predicting the severity of side effects among university students receiving COVID-19 vaccines. BMC Infect Dis 2023; 23: 378.
8.
Chakraborty C, Sharma A, Bhattacharya M, Lee S. A detailed overview of immune escape, antibody escape, partial vaccine escape of SARS-CoV-2 and their emerging variants with escape mutations. Front Immunol 2022; 13: 801522.
9.
Chakraborty C, Bhattacharya M, Sharma A, et al. Immediate need for next-generation and mutation-proof vaccine to protect against current emerging Omicron sublineages and future SARS-CoV-2 variants: an urgent call for researchers and vaccine companies – correspondence. Int J Surg 2022; 106: 106903.
10.
Bhattacharya M, Chatterjee S, Sharma A, Lee S, Chakraborty C. Delta variant (B.1.617.2) of SARS-CoV-2: current understanding of infection, transmission, immune escape, and mutational landscape. Folia Microbiol 2022; 68: 17-28.
11.
Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727-33.
12.
World Health Organization. Coronavirus disease (COVID-19) outbreak (https:// www.who.int).
13.
Mtei M, Mboya IB, Mgongo M, et al. Confidence in COVID-19 vaccine effectiveness and safety and its effect on vaccine uptake in Tanzania: a community-based cross-sectional study. Hum Vaccin Immunother 2023; 19: 2191576.
14.
World Health Organization. COVID-19 advice for the public: getting vaccinated. Geneva (Switzeland): World Health Organization 2022 [accessed 2022 Nov 24].
15.
Our World In Data. Total Covid-19 doses administered per 100 people. England and Wales: Our World In Data 2022.
16.
Majidpoor J, Mortezaee K. Interleukin-6 in SARS-CoV-2 induced disease: interactions and therapeutic applications. Biomed Pharmacother 2022; 145: 112419.
17.
He Q, Wu L, Xu Z, et al. An updated atlas of antibody evasion by SARS-CoV-2 Omicron sub-variants including BQ.1.1 and XBB. Cell Rep Med 2023; 4: 100991.
18.
He C, Ali A, Lei H, et al. A recombinant spike-XBB.1.5 protein vaccine induces broad-spectrum immune responses against XBB.1.5-included Omicron variants of SARS-CoV-2. MedComm (2020) 2023; 4: e263.
19.
Revel M, Parkar A, Prosch H, et al. COVID-19 patients and the radiology department - advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). Eur Radiol 2020; 30: 4903-9.
20.
Yang R, Li X, Liu H, et al. Chest CT severity score: an imaging tool for assessing severe COVID-19. Radiol Cardiothorac Imaging 2020; 2: e200047.
21.
Mehta P, McAuley D, Brown M, Sanchez E, Tattersall R, Manson J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033-4.
22.
Jamilloux Y, Henry T, Belot A, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev 2020; 19: 102567.
23.
Sun X, Wang T, Cai D, et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev 2020; 53: 38-42.
24.
Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol 2021; 93: 250-6.
25.
Barnes E, Goodyear CS, Willicombe M, et al. SARS-CoV-2-specific immune responses and clinical outcomes after COVID-19 vaccination in patients with immune-suppressive disease. Nat Med 2023; 29: 1760-74.
26.
Zhao N, Zhang T, Zhao Y, Zhang J, Wang K. CD3+T, CD4+T, CD8+T, and CD4+T/CD8+T ratio and quantity of T cells in peripheral blood of HIV-infected/AIDS patients and its clinical significance. Comput Math Methods Med 2021; 2021: 8746264.
27.
Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A. Type 1 diabetes mellitus as a disease of the -cell (do not blame the immune system?). Nat Rev Endocrinol 2021; 17: 150-61.
28.
Sestan M, Marinović S, Kavazović I, et al. Virus-induced interferon- causes insulin resistance in skeletal muscle and derails glycemic control in obesity. Immunity 2018; 49: 164-77.e6.
29.
Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol 2021; 17: 11-30.
30.
Awatade NT, Wark PA, Chan AS, et al. The Complex Association between COPD and COVID-19. J Clin Med 2023; 12: 3791.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.