Introduction
Gastric cancer is one among the frequently detected malignancies throughout the world, and its incidence has increased over the past decade [1, 2]. Despite advancement in the field of chemotherapy and technology, gastric cancer is the third leading cause of death associated with cancer globally [3, 4]. The highest incidence of gastric cancer has been reported in the East Asia [3, 4]. The high mortality rate of gastric cancer patients is associated with its detection at advanced stage in most cases [5]. The prognosis of gastric cancer patients is very poor [6]. The most common hindrance to the gastric cancer treatment strategies is the high rate of infiltration and invasion [7]. Therefore, the discovery of chemotherapeutic agents that can effectively inhibit proliferation and metastasis of gastric cancer cells is urgently needed.
MicroRNAs (miRNAs) usually comprise ~19–25 nucleotides and exhibit their effect by regulating gene expression for inhibiting or cleaving the translation through involvement of one or more mRNAs [8, 9]. The miRNAs regulate cellular proliferation, death, and development of various organs in the body [10, 11]. It has been shown that miRNAs play an important role in the regulation of gastric cancer development and progression [12, 13]. The expression of miR-145 was found to be down-regulated in cancerous colorectal lesions [14]. Studies have shown that miR-145 acts as a tumour suppressor molecule because it regulates tumour cell growth by targeting expression of Myc proto-oncogene protein (c-Myc) and other transcription factors [15, 16]. In gastric cancer cells, overexpression of miR-145 has been reported to inhibit growth and metastasis through targeting MYO6 expression [17, 18]. Another study has revealed that miR-145 suppresses proliferation, inhibits metastasis, and causes arrest of cell cycle in gastric cancer cells by down-regulation of Sp1 expression [19]. Down-regulation of N-cadherin translation by miR-145 has also been found to inhibit metastasis of gastric cancer cells [20]. Therefore, miR-145 employs diverse mechanisms to suppress the proliferation of gastric cancer cells. In the present study the effect of 5,7,2,5-tetrahydroxy-8,6-dimethoxyflavone (THDMF) on gastric cancer cells was investigated and the underlying mechanism was explored. The study demonstrated that THDMF caused gastric cancer cell apoptosis, led to arrest of cell cycle, and inhibited proliferation. The THDMF exposure of MKN28 cells promoted expression of miR-145 and down-regulated PI3K/AKT signalling pathway.
Material and methods
Cell culture
The human gastric cancer cell lines MKN28 and MKN45, and the immortalised human gastric epithelial mucosa cell line GES-1 were supplied by the Shanghai Tumour Research Institute, Shanghai, China. The cell lines were grown in DMEM mixed with 10% foetal bovine serum. The medium also contained 100 U/ml penicillin and 100 μg/ml streptomycin. The cell lines were cultured under a humidified atmosphere of 5% CO2 atmosphere in an incubator at 37°C.
Cell proliferation assay
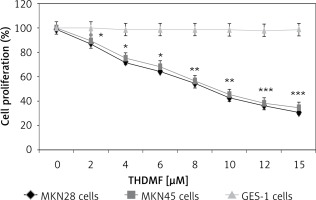
The proliferation of MKN28, MKN45, and GES-1 cells after 72 h of treatment with THDMF or dimethyl sulfoxide alone (control) was measured by MTT colorimetric assays. The cell lines were exposed to 2, 4, 6, 8, 10, 12, and 15 μM of THDMF at 1 × 105 cells/well density in 96-well plates for 72 h. Then 5 mg/ml solution of MTT (20 μl) was added to each well of the plate and cells were incubated for a further 4 h. Then the medium in the wells was discarded and dimethyl sulphoxide (150 μl) was placed in each well to dissolve any solid crystal that had formed. The plates were kept in shaker for 20 min at room temperature before the optical density was recorded at 490 nm in a scanning multi-well spectrophotometer.
Apoptosis analysis
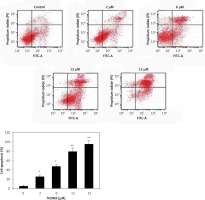
The MKN28 cells were put into six-well plates and grown for 24 h before incubation with 2, 6, 12, and 15 μM of THDMF for 72 h. The cells were harvested and centrifuged for 20 min, and the cell pellets were twice washed with cold PBS. After washing, the cells were subjected to incubation under complete darkness with 5 μl of PI at room temperature for 20 min. The cells in each tube were then treated with 350 μl of 1X binding buffer for 15 min. The cell apoptosis was analysed by flow cytometry using a BD Accuri™ C6 Flow cytometer (BD Biosciences, San Jose, CA, USA).
Cell cycle analysis
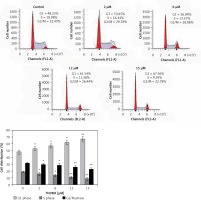
MKN28 cells were put at 2 × 105 cells per well density in 60 mm plates and treated with 2, 6, 12, and 15 μM of THDMF for 72 h. The cells after trypsinisations were washed twice with PBS and subsequently fixed with 70% cold ethyl alcohol overnight. Then the cells were treated with RNase A (20 μg/ml) at 37°C before staining with PI (10 μg/ml) at 37°C. The flow cytometry employing a FACS-Calibur instrument (BD Biosciences, San Jose, CA, USA) was used for analysis of DNA content and cell cycle distribution.
Cell invasion assay
The 24-well Transwell plates (8 mm pore size) were subjected to coating with 200 mg/ml Matrigel and then dried under sterile conditions overnight. MKN28 cells were exposed to 2, 6, 12, and 15 μM of THDMF at 2 × 105 cells/ml concentration in RPMI-1640 medium on the upper chamber. The lower chamber contained RPMI-1640 medium mixed with 20% FBS. Following incubation for 72 h, the non-invasive cells on the upper chamber were cleaned using cotton wool. The cells were then fixed in methyl alcohol for 15 min at room temperature followed by staining with haematoxylin-eosin for 25 min. A light microscope (Olympus Corporation, Tokyo, Japan) was used to count the number of cells that invaded the lower chamber.
In vitro wound healing assay
The MKN28 cells were put at 2 × 105 cells/ml density in a six-well plate and allowed to attain 100% confluence by incubation at 37°C. The cells were starved for 24 h, and then a 100 ml plastic pipette tip was used to draw a wound (straight cell-free) through the middle of the wells. The wells were washed with PBS two times followed by treatment with 2, 6, 12, and 15 μM of THDMF for 72 h. The cells, after fixing and staining with 3.5% ethyl alcohol containing 1.5% crystal violet dye for 15 min, were observed for migration potential. An inverted light microscope (Nikon Corporation) was used to observe the cells at five randomly selected fields.
Western blot analysis
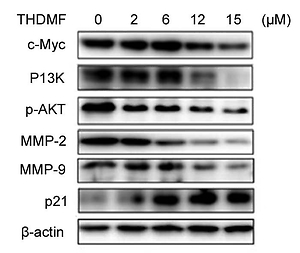
The MKN28 cells, following 72 h of exposure to 2, 6, 12, and 15 μM of THDMF, were collected and then lysed on treatment with lysis buffer (Tris-HCl (40 mM; pH 7.5) mixed with NaCl (150 mM) and Triton X-100 (1%; v/v) and protease inhibitors). The lysate was freed from cell debris by centrifugation for 20 min at 1200 g to obtain the supernatants. The concentration of proteins was measured using a commercially available bicinchoninic acid protein assay kit in accordance with the instructions from the manufacturer. The 20 μg per lane protein samples were resolved using 10% SDS-PAGE transferred subsequently onto the polyvinylidene difluoride membranes. The membranes were prior blocked by incubation with 5% skimmed milk and Tris buffered saline containing Tween-20 (0.1%) for 2 h at room temperature. The samples were probed by incubation with primary antibodies; anti-c-Myc, anti-p-AKT, anti-PI3K, anti-P21, anti-MMP-2, anti-MMP-9, and anti-GAPDH. The membranes, after washing with 1X PBST three times, were subjected to incubation for 2 h with horseradish peroxidase-conjugated secondary antibody at room temperature. The immunoreactive band visualisation was performed using Signal Fire™ Plus ECL Reagent (cat no. 12630; Cell Signalling Technology, Inc.) using GAPDH as a loading control. The quantification of intensities was performed using Image J version 2.0 software (Bio-Rad Laboratories Inc., USA).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from MKN28 cells following 72 h of exposure to 2, 6, 12, and 15 μM of THDMF was extracted by TRIzol reagent according to manual protocol. The M-MLV and SYBR Green master mix kit were used for reverse-transcription and cDNA amplification, respectively, in accordance with the manufacturer’s protocol. Amplification of the genes was performed using specific oligonucleotide primers and GAPDH as an endogenous control. The primers used were: miR-145 forward: 5’-GTC CAG TTT TCC CAG GAA TCC CT-3’; and backward: 5’-GCT GTC AAC GAT ACG CTA CCT A-3’. The comparative Ct method (2–ΔΔCt) was used for the analysis of data. The experiments were carried out separately in triplicate for each clone. The conditions employed for amplification were: 93°C for 5 min, 40 cycles at 93°C for 30 s, 54°C for 30 s, 70°C for 30 s, and 70°C for 10 min.
Statistical analysis
The data expressed are the mean ± standard deviation (SD) of experiments carried out independently in triplicate. The data were analysed using SPSS version 17.0 software (SPSS, Inc., Chicago, IL, USA). The one-way analysis of variance followed by Tukey’s post-hoc test was used for analysis of the data. At p < 0.05 differences were taken as statistically significant.
Results
THDMF specifically inhibits MKN28 and MKN45 gastric cancer cell proliferation
Exposure of MKN28 and MKN45 cells to THDMF significantly (p < 0.05) reduced proliferation but did not affect the normal gastric GES-1 cell viability (Figure 1). Treatment of MKN28 cells with THDMF at 2, 4, 6, 8, 10, 12, and 15 μM reduced proliferation to 87, 72, 64, 55, 43, 36, and 31%, respectively. The proliferation of MKN45 cells was decreased to 89, 75, 68, 56, 45, 38, and 34%, respectively, on exposure to 2, 4, 6, 8, 10, 12, and 15 μM of THDMF for 72 h. However, no change in GES-1 cell proliferation was caused by THDMF in the concentration range of 2–15 μM.
THDMF promotes MKN28 cell apoptosis
The apoptosis induction in MKN28 cells was assessed on exposure to 2, 6, 12, and 15 μM of THDMF at 72 h (Figure 2). The data from flow cytometry showed a significant increase in early as well as late apoptotic cell population in MKN28 cell cultures on exposure to THDMF. Exposure of MKN28 cells to 15 μM of THDMF raised the apoptotic cell percentage to 47.68% in comparison to 2.46% in control cells.
THDMF induces MKN28 cell cycle arrest
The changes in MKN28 cell cycle progression by 2, 6, 12, and 15 μM of THDMF were detected by flow cytometry (Figure 3). Exposure of MKN28 cells to THDMF for 72 h increased the proportion of cells in the G1 phase of the cell cycle. The cell percentage in the G1 phase increased to 67.36% on exposure to 15 μM of THDMF in comparison to 48.25% in control cells. The population of cells in the S and G2/M phases was significantly (p < 0.02) decreased on exposure to THDMF.
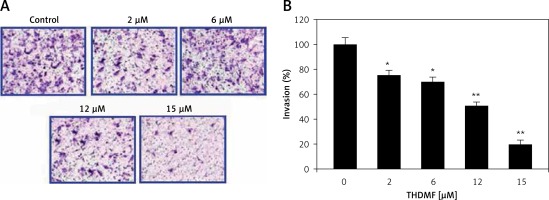
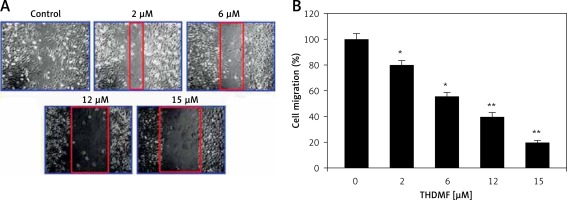
THDMF inhibits MKN28 cell invasion and migration
The data from Matrigel Transwell assay showed a significant (p < 0.05) decrease in invasion of MKN28 cells on exposure to THDMF (Figure 4). Exposure of MKN28 cells to 2, 6, 12, and 15 μM of THDMF for 72 h led to concentration-based reduction of cell invasion. The invasion of MKN28 cells was decreased eight-fold on exposure to 15 μM of THDMF. The migration potential of MKN28 cells was also reduced significantly (p < 0.05) on exposure to 2, 6, 12, and 15 μM of THDMF (Figure 5). The wound healing assay revealed that reduction of MKN28 cell migration potential was significant from 2 μM and maximum at 15 μM of THDMF.
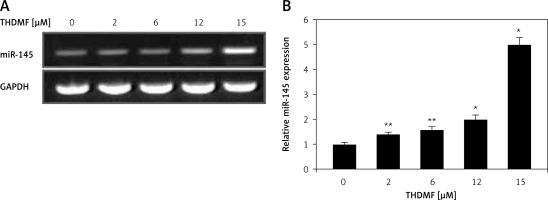
THDMF promotes miR-145 expression in MKN28 cells
The miR-145 expression in MKN28 cells was markedly increased on exposure to 2, 6, 12, and 15 μM of THDMF at 72 h (Figure 6). The RT-qPCR showed that the increase in miR-145 expression was significant in MKN28 cells treated with 2 μM of THDMF. A dose-based promotion in miR-145 expression was detected with the increase in THDMF concentration from 2 to 15 μM.
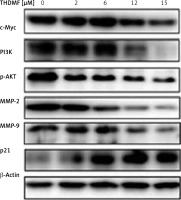
THDMF regulates PI3K/AKT signalling pathway in MKN28 cells
Western blotting showed that exposure of MKN28 cells to THDMF suppressed c-Myc, PI3K, p-AKT, MMP2, and MMP9 expression (Figure 7). There was a concentration-based reduction of PI3K, c-Myc, MMP2/-9, and p-AKT expression in MKN28 cells on exposure to THDMF. However, the expression of p21 protein in MKN28 cells was markedly promoted on exposure to THDMF.
Discussion
The present study investigated the anti-cancer potential of THDMF against gastric cancer cells in vitro and explored the molecular mechanism involved. The data showed that THDMF caused apoptosis activation and cell cycle arrest, leading to suppression of gastric cancer cell proliferation. The anti-proliferative action of THDMF was associated with the promotion of miR-145 expression and regulation of PI3K/AKT signalling pathway. As the expression of miR-145 in immortalised human gastric epithelial mucosa cells, GES-1 is higher compared to cancer cell lines; therefore, GES-1 cells were insensitive to THDMF exposure.
Gastric cancer is a commonly detected malignancy, diagnosed in around one million people every year throughout the world [21]. In the present study THDMF exposure significantly reduced MKN28 and MKN45 gastric cancer cell proliferation without affecting the normal gastric GES-1 cell viability. Moreover, THDMF caused apoptosis activation and arrest of cell cycle in the G1 phase in MKN28 cells. It has been found that expression of miR-145 is down-regulated in various types of cancer cells and is therefore considered to be an important target for chemotherapeutic intervention [22–25]. It is believed that miR-145 plays an important role as the tumour suppressor factor by inhibition of carcinoma cell proliferation [18–20]. The up-regulation of miR-145 expression in cancer cells inhibits proliferation and causes arrest of cell cycle in the G1 phase [26]. It was demonstrated that HuR expression is elevated in gastric cancer tissues as well as cell lines, and this over-expression has a significant relationship with lymph node metastasis. Targeting HuR by RNA interference inhibited viability and enhanced apoptosis via promotion of pro-apoptotic factors (Bcl-2 and Bax). Moreover, HuR expression was found to be inversely correlated with miR-145 expression in gastric cancer tissues, and HuR was shown to be a direct target of miR-145 [27]. The MKN45 cell proliferative potential and metastasis is inhibited by lidocaine through up-regulation of miR-145 [28]. The present study showed down-regulation of miR-145 expression in MKN28 gastric cancer cells in consistence with the reported literature [18]. However, treatment of MKN28 cells with THDMF markedly promoted the expression of miR-145 in a dose-based manner. These findings suggest that THDMF exhibits cytotoxicity effect on MKN28 cells through up-regulation of miR-145 expression.
The oncogene c-Myc is linked to the uncontrolled proliferation, regulation of cell apoptosis, and transformation of cells [29]. The higher levels of c-Myc generate apoptotic signals leading to DNA damage in the epithelial cells [30]. It has been found that inhibition of c-Myc expression plays a vital role in the arrest of cell cycle [31]. In the present study THDMF treatment of MKN28 cells markedly down-regulated the expression of c-Myc. Exposure of MKN28 cells to THDMF led to arrest of cell cycle in the G1 phase. The data from flow cytometry showed that THDMF exposure of MKN28 cells markedly increased the MKN28 cell percentage in the G1 phase and decreased the population in S and G2/M phases. Therefore, THDMF arrested MKN28 cell cycle in G1 phase through down-regulation of c-Myc expression. The PI3K/AKT pathway regulates metastasis, proliferation, and apoptosis of the cells through inhibition of down-stream phosphorylation [32, 33]. In the present study THDMF exposure of MKN28 cells suppressed the PI3K and p-AKT expression. Thus, THDMF treatment regulates the PI3K/AKT signalling pathway in MKN28 cancer cells. The most important members of metalloproteinases are MMP-2 and MMP-9, which play a leading role in invasion, metastasis, and vascularisation of the tumour cell through degradation of type IV collagen [34]. The results from the present study showed that exposure of MKN28 cells to THDMF down-regulated the levels of MMP-2 and MMP-9. Thus, THDMF inhibited the invasion and migration of MKN28 cells through suppression of MMP-2 and MMP-9 levels.
In conclusion, the present study demonstrated that THDMF exhibits an anti-cancer effect on gastric cancer cells in vitro through apoptosis activation and cell cycle arrest. Moreover, the anti-proliferative action of THDMF involved promotion of miR-145 expression and down-regulation of PI3K/AKT signalling pathway. Therefore, THDMF may be utilised as a potential novel therapeutic agent for the treatment of gastric cancer.