Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
Editor's Choice
NUTRITION / CLINICAL RESEARCH
2024 update on postmarketing nutrivigilance safety profile: a line of dietary food supplements containing red yeast rice for dyslipidemia
1
Department of Preventive Cardiology and Lipidology, Medical University of Lodz (MUL), Lodz, Poland
2
Department of Cardiology and Adult Congenital Heart Diseases, Polish Mother’s Memorial Hospital Research Institute (PMMHRI), Lodz, Poland
3
Cardiovascular Research Centre, University of Zielona Gora, Zielona Gora, Poland
4
Department of Nutritional Sciences and Dietetics, International Hellenic University, Thessaloniki, Greece
5
School of Medicine, European University Cyprus, Nicosia, Cyprus
6
Institute of Cardiology and Regenerative Medicine, Faculty of Medicine, University of Latvia, Riga, Latvia
7
Pauls Stradins Clinical University Hospital, Riga, Latvia
8
Department of Cardiology, "Victor Babes" University of Medicine and Pharmacy, Timisoara, Romania
9
Institutul de Boli Cardiovasculare, Timisoara, Romania
10
Cardiology Department, Hospital Universitario La Paz, Madrid, Spain
11
2nd Department of Cardiology of the East Slovak Institute of Cardiovascular Disease and Faculty of Medicine PJ Safarik University, Kosice, Slovak Republic
12
School of Pharmacy and Biomolecular Sciences, Liverpool John Moores University, Liverpool, UK
13
Liverpool Centre for Cardiovascular Science, Liverpool, UK
14
Hypertension and Cardiovascular Risk Factors Research Center, Medical and Surgical Sciences Department, Sant'Orsola-Malpighi University Hospital, Bologna, Italy
15
Cardiovascular Medicine Unit, Heart, Thoracic and Vascular Department, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
Submission date: 2024-02-07
Final revision date: 2024-05-29
Acceptance date: 2024-06-16
Online publication date: 2024-06-16
Corresponding author
Maciej Banach
Department of Preventive Cardiology and Lipidology, Medical University of Lodz, Rzgowska 281/289, 93-338 Lodz, Poland Phone: +48422711124
Department of Preventive Cardiology and Lipidology, Medical University of Lodz, Rzgowska 281/289, 93-338 Lodz, Poland Phone: +48422711124
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Considering lack of a European standardized postmarketing food supplement surveillance system (nutrivigilance), some member states and companies have developed their own approaches to monitoring potential adverse reactions (AEs) to secure a high level of product safety. This paper updates 2021 results of the use of a nutrivigilance system (which contained data to the end of 2019) in monitoring the incidence of spontaneously reported suspected AEs associated with red yeast rice (RYR) containing food supplements.
Material and methods:
We report the data from a widely used product marketed under the trademark Armolipid/Armolipid Plus. Postmarketing information was collected in a voluntary nutrivigilance system established by the manufacturing company (Meda Pharma SpA, a Viatris Company, Monza, Italy). From 1st October 2004 to 31st December 2023, this system captured cases of suspected adverse reactions spontaneously reported by consumers, healthcare professionals, health authorities, regardless of causality.
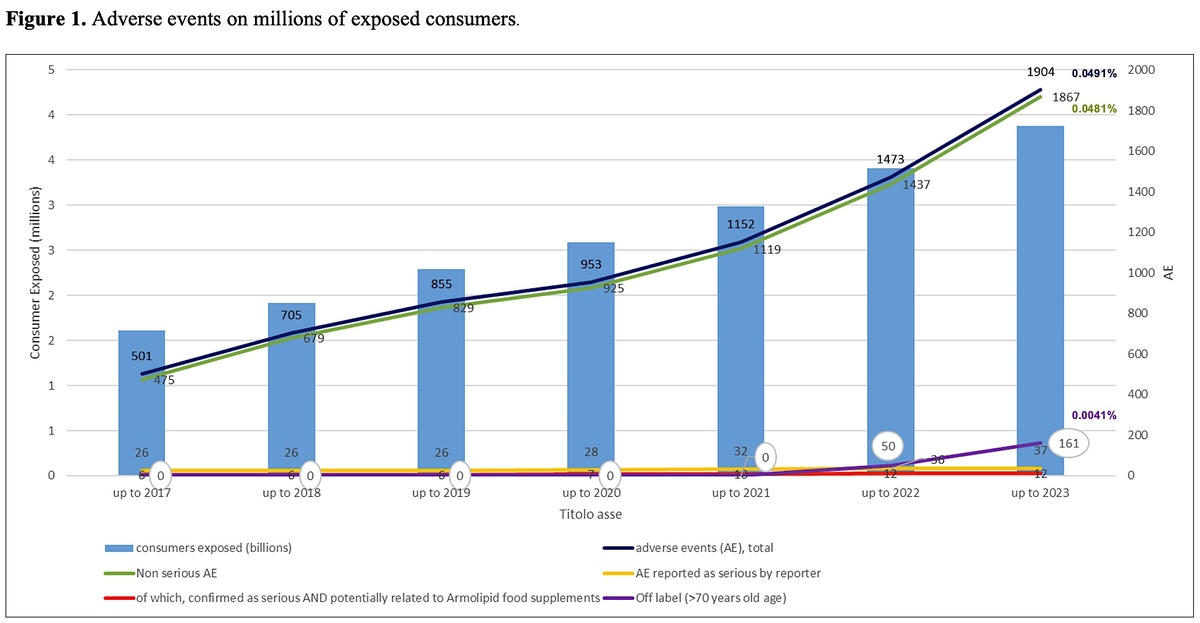
Results:
The total number of case reports received mentioning the RYR food supplement product line increased to 1186, in which 1904 adverse events (AEs) were reported. The total reporting rate of AEs was estimated to be 0.049% of 3,880,865 exposed consumers. Of the 1186 cases, 28 (0.0007% of exposed consumers) included suspected serious adverse events (SAEs). After very careful investigation, 9 cases (0.0002% of consumers exposed) and 12 AEs were assessed by the manufacturer as serious and potentially related to exposure to the above-mentioned RYR-based nutraceutical. Off-label reports linked to the newly introduced limitation at 70 years of age were observed, in contrast to the previous analysis.
Conclusions:
This updated nutrivigilance-derived data analysis confirms a very low incidence of suspected AEs associated with the RYR product line. Consumer safety of food supplements could be generally improved by raising awareness of the importance of following the indications and warnings detailed in a food supplement’s labelling. Changes to the exposed population may impact the reporting rates.
Considering lack of a European standardized postmarketing food supplement surveillance system (nutrivigilance), some member states and companies have developed their own approaches to monitoring potential adverse reactions (AEs) to secure a high level of product safety. This paper updates 2021 results of the use of a nutrivigilance system (which contained data to the end of 2019) in monitoring the incidence of spontaneously reported suspected AEs associated with red yeast rice (RYR) containing food supplements.
Material and methods:
We report the data from a widely used product marketed under the trademark Armolipid/Armolipid Plus. Postmarketing information was collected in a voluntary nutrivigilance system established by the manufacturing company (Meda Pharma SpA, a Viatris Company, Monza, Italy). From 1st October 2004 to 31st December 2023, this system captured cases of suspected adverse reactions spontaneously reported by consumers, healthcare professionals, health authorities, regardless of causality.
Results:
The total number of case reports received mentioning the RYR food supplement product line increased to 1186, in which 1904 adverse events (AEs) were reported. The total reporting rate of AEs was estimated to be 0.049% of 3,880,865 exposed consumers. Of the 1186 cases, 28 (0.0007% of exposed consumers) included suspected serious adverse events (SAEs). After very careful investigation, 9 cases (0.0002% of consumers exposed) and 12 AEs were assessed by the manufacturer as serious and potentially related to exposure to the above-mentioned RYR-based nutraceutical. Off-label reports linked to the newly introduced limitation at 70 years of age were observed, in contrast to the previous analysis.
Conclusions:
This updated nutrivigilance-derived data analysis confirms a very low incidence of suspected AEs associated with the RYR product line. Consumer safety of food supplements could be generally improved by raising awareness of the importance of following the indications and warnings detailed in a food supplement’s labelling. Changes to the exposed population may impact the reporting rates.
REFERENCES (30)
1.
Mensah GA, Fuster V, Murray CJL, Roth GA; Global Burden of Cardiovascular Diseases and Risks Collaborators. Global burden of cardiovascular diseases and risks, 1990-2022. J Am Coll Cardiol 2023; 82: 2350-473.
2.
Banach M, Surma S. A look to the past – what has had the biggest impact on lipids in the last four decades? A personal perspective. Arch Med Sci 2023; 19: 559-64.
3.
Penson PE, Pirro M, Banach M. LDL-C: lower is better for longer-even at low risk. BMC Med 2020; 18: 320.
4.
Banach M, Burchardt P, Chlebus K, et al. PoLA/CFPiP/PCS/PSLD/PSD/PSH guidelines on diagnosis and therapy of lipid disorders in Poland 2021. Arch Med Sci 2021; 17: 1447-547.
5.
Ge L, Sadeghirad B, Ball GDC, et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials. BMJ 2020; 369: m696.
6.
Mach F, Baigent C, Catapano AL, et al; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020; 41: 111-88.
7.
Cicero AFG, Colletti A, Bajraktari G, et al. Lipid lowering nutraceuticals in clinical practice: position paper from an International Lipid Expert Panel. Arch Med Sci 2017; 13: 965-1005.
8.
Banach M, Patti AM, Giglio RV, et al. The role of nutraceuticals in statin intolerant patients. J Am Coll Cardiol 2018; 72: 96-118.
9.
Banach M, Catapano AL, Cicero AFG, et al.; Behalf Of The International Lipid Expert Panel ILEP. Red yeast rice for dyslipidaemias and cardiovascular risk reduction: a position paper of the International Lipid Expert Panel. Pharmacol Res 2022; 183: 106370.
10.
Banach M, Bruckert E, Descamps OS, et al. The role of red yeast rice (RYR) supplementation in plasma cholesterol control: a review and expert opinion. Atheroscler Suppl 2019; 39: e1-8.
11.
Mazidi M, Kengne AP, Banach M; Lipid and Blood Pressure Meta-analysis Collaboration Group. Effects of coenzyme Q10 supplementation on plasma C-reactive protein concentrations: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res 2018; 128: 130-6
12.
Banach M, Serban C, Sahebkar A, et al. Effects of coenzyme Q10 on statin-induced myopathy: a meta-analysis of randomized controlled trials. Mayo Clin Proc 2015; 90: 24-34.
13.
EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), Younes M, Aggett P, et al. Scientific opinion on the safety of monacolins in red yeast rice. EFSA J 2018; 16: e05368.
14.
Fogacci F, Banach M, Mikhailidis DP, et al. Safety of red yeast rice supplementation: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res 2019; 143: 1-16.
15.
Mazzanti G, Moro PA, Raschi E. Adverse reactions to dietary supplements containing red yeast rice: assessment of cases from the Italian surveillance system. Br J Clin Pharmacol 2017; 83: 894-908.
16.
Banach M, Norata GD. Rhabdomyolysis or severe acute hepatitis associated with the use of red yeast rice extracts: an update from the adverse event reporting systems. Curr Atheroscler Rep 2023; 25: 879-88.
17.
Norata GD, Banach M. The impact of red yeast rice extract use on the occurrence of muscle symptoms and liver dysfunction: an update from the adverse event reporting systems and available meta-analyses. Nutrients 2024; 16: 444.
18.
Cohen PA, Avula B, Khan IA. Variability in strength of red yeast rice supplements purchased from mainstream retailers. Eur J Prev Cardiol 2017; 24: 1431-4.
19.
Lu Z, Kou W, Du B, et al. Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. Am J Cardiol 2008; 101: 1689-93.
20.
Gheith O, Sheashaa H, Abdelsalam M. Efficacy and safety of Monascus purpureus Went rice in subjects with secondary hyperlipidemia. Clin Exp Nephrol 2008; 12: 189-94.
21.
Lin CC, Li TC, Lai MM. Efficacy and safety of Monascus purpureus Went rice in subjects with hyperlipidemia. Eur J Endocrinol 2005; 153: 679-86.
22.
Affuso F, Ruvolo A, Micillo F. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr Metab Cardiovasc Dis 2010; 20: 656-61.
23.
Affuso F, Mercurio V, Ruvolo A, et al. A nutraceutical combination improves insulin sensitivity in patients with metabolic syndrome. World J Cardiol 2012; 4: 77-83.
24.
Barrios V, Escobar C, Cicero AF, et al. A nutraceutical approach (Armolipid Plus) to reduce total and LDL cholesterol in individuals with mild to moderate dyslipidemia: review of the clinical evidence. Atheroscler Suppl 2017; 24: 1-15.
25.
Banach M, Katsiki N, Latkovskis G, et al. Postmarketing nutrivigilance safety profile: a line of dietary food supplements containing red yeast rice for dyslipidemia. Arch Med Sci 2021; 17: 856-63.
26.
The use of the WHO-UMC system for standardised case causality assessment. Uppsala Monitoring Centre. www.who-umc.org/media/164200/who-umc-causality-assessment_new-logo.pdf. Published 2018. Accessed May 29, 2023.
27.
Penson PE, Mancini GBJ, Toth PP, et al. Introducing the ‘Drucebo’ effect in statin therapy: a systematic review of studies comparing reported rates of statin-associated muscle symptoms, under blinded and open-label conditions. J Cachexia Sarcopenia Muscle 2018; 9: 1023-33.
28.
Penson PE, Bruckert E, Marais D, et al.; International Lipid Expert Panel (ILEP). Step-by-step diagnosis and management of the nocebo/drucebo effect in statin-associated muscle symptoms patients: a position paper from the International Lipid Expert Panel (ILEP). J Cachexia Sarcopenia Muscle 2022; 13: 1596-622.
29.
Fogacci F, Giovannini M, D’Addato S, Grandi E, Cicero AFG. Effect of dietary supplementation with a new nutraceutical formulation on cardiometabolic risk factors: a double-blind, placebo-controlled, randomized clinical study. Arch Med Sci Atheroscler Dis 2023; 8: e53-9.
30.
Cicero AFG, Fogacci F, Tocci G, et al. Three arms, double-blind, non-inferiority, randomized clinical study testing the lipid-lowering effect of a novel dietary supplement containing red yeast rice and artichoke extracts compared to Armolipid Plus® and placebo. Arch Med Sci 2023; 19: 1169-79.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.