Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
Editor's Choice
NUTRITION / RESEARCH PAPER
2024 Update on Postmarketing Nutrivigilance Safety Profile: A Line of Dietary Food Supplements Containing Red Yeast Rice for Dyslipidemia.
1
Department of Preventive Cardiology and Lipidology, Medical University of Lodz (MUL), Lodz, Poland, Poland
2
Department of Cardiology and Adult Congenital Heart Diseases, Polish Mother’s Memorial Hospital Research Institute (PMMHRI), Lodz, Poland, Poland
3
Cardiovascular Research Centre, University of Zielona Gora, Zielona Gora, Poland, Poland
4
Department of Nutritional Sciences and Dietetics, International Hellenic University, Thessaloniki, Greece, Greece
5
School of Medicine, European University Cyprus, Nicosia, Cyprus, Cyprus
6
Institute of Cardiology and Regenerative Medicine, Faculty of Medicine, University of Latvia, Riga, Latvia, Latvia
7
Pauls Stradins Clinical University Hospital, Riga, Latvia, Latvia
8
Department of Cardiology, "Victor Babes" University of Medicine and Pharmacy, Timisoara, Romania, Romania
9
Institutul de Boli Cardiovasculare, Timisoara, Romania, Romania
10
Cardiology Department, Hospital Universitario La Paz, Madrid, Spain, Spain
11
2nd Department of Cardiology of the East Slovak Institute of Cardiovascular Disease and Faculty of Medicine PJ Safarik University, Kosice, Slovak Republic, Slovak Republic
12
School of Pharmacy and Biomolecular Sciences, Liverpool John Moores University, Liverpool, UK, United Kingdom
13
Liverpool Centre for Cardiovascular Science, Liverpool, UK, United Kingdom
14
Hypertension and Cardiovascular Risk Factors Research Center, Medical and Surgical Sciences Department, Sant'Orsola-Malpighi University Hospital, Bologna, Italy, Italy
15
Cardiovascular Medicine Unit, Heart, Thoracic and Vascular Department, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy., Italy
Submission date: 2024-02-07
Final revision date: 2024-05-29
Acceptance date: 2024-06-16
Online publication date: 2024-06-16
Corresponding author
Maciej Banach
Department of Preventive Cardiology and Lipidology, Medical University of Lodz (MUL), Lodz, Poland, Lodz, Poland
Department of Preventive Cardiology and Lipidology, Medical University of Lodz (MUL), Lodz, Poland, Lodz, Poland
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Considering lack of a European standardized postmarketing food supplement surveillance system, some member states and companies have developed their own approaches to monitoring potential AEs to secure a high level of product safety. This paper updates 2021 results of the use of a nutrivigilance system in monitoring the incidence of spontaneously reported suspected AEs associated with RYR-containing food supplements.
Material and methods:
We report the data from a product marketed under the trademark Armolipid/Armolipid Plus. Postmarketing information was collected in a voluntary nutrivigilance system established by the manufacturing company (Meda Pharma SpA, a Viatris Company, Monza, Italy). From 1st October 2004-31st December 2023, this system captured cases of suspected AEs spontaneously reported by consumers, healthcare professionals, health authorities, regardless of causality.
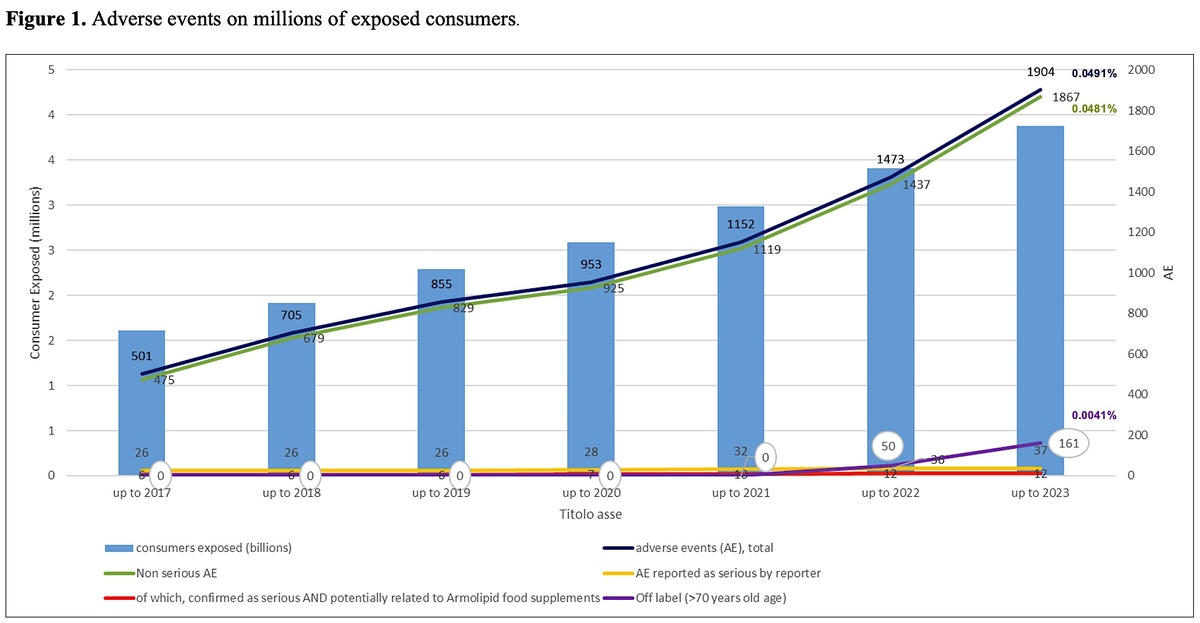
Results:
The total number of case reports received mentioning the RYR-food supplement product line increased to 1186, in which 1904 AEs were reported. The total reporting rate of AEs was estimated to be 0.049% of 3,880,865 exposed consumers. Of the 1186 cases, 28 (0.0007%) included suspected SAEs. After careful investigation, 9 cases (0.0002%) and 12 AEs were assessed by the manufacturer as serious and potentially related to exposure to the above-mentioned RYR-based nutraceutical. Off-label reports linked to the newly introduced limitation at 70 years of age were observed, in contrast to the previous analysis.
Conclusions:
These updated results confirm a very low incidence of RYR suspected AEs. Consumer safety of food supplements could be generally improved by raising awareness of the importance of following the indications and warnings detailed in a food supplement’s labelling.
Considering lack of a European standardized postmarketing food supplement surveillance system, some member states and companies have developed their own approaches to monitoring potential AEs to secure a high level of product safety. This paper updates 2021 results of the use of a nutrivigilance system in monitoring the incidence of spontaneously reported suspected AEs associated with RYR-containing food supplements.
Material and methods:
We report the data from a product marketed under the trademark Armolipid/Armolipid Plus. Postmarketing information was collected in a voluntary nutrivigilance system established by the manufacturing company (Meda Pharma SpA, a Viatris Company, Monza, Italy). From 1st October 2004-31st December 2023, this system captured cases of suspected AEs spontaneously reported by consumers, healthcare professionals, health authorities, regardless of causality.
Results:
The total number of case reports received mentioning the RYR-food supplement product line increased to 1186, in which 1904 AEs were reported. The total reporting rate of AEs was estimated to be 0.049% of 3,880,865 exposed consumers. Of the 1186 cases, 28 (0.0007%) included suspected SAEs. After careful investigation, 9 cases (0.0002%) and 12 AEs were assessed by the manufacturer as serious and potentially related to exposure to the above-mentioned RYR-based nutraceutical. Off-label reports linked to the newly introduced limitation at 70 years of age were observed, in contrast to the previous analysis.
Conclusions:
These updated results confirm a very low incidence of RYR suspected AEs. Consumer safety of food supplements could be generally improved by raising awareness of the importance of following the indications and warnings detailed in a food supplement’s labelling.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.