Recent data clearly show that we are extremely ineffective in the use of lipid-lowering therapy (LLT). Furthermore, the problem seems to be the most serious in the group of patients at very high and extremely high-risk – the patients who stand to benefit most from effective therapy [1]. The results of the DaVinci study showed that the proportion of very high-risk patients reaching the low-density lipoprotein cholesterol (LDL-C) goal (< 55 mg/dl; 1.4 mmol/l) is only 18% in Europe [2], and only 13% in Central and Eastern European (CEE) countries [3]. Only a very small percentage of patients at extremely high-risk, reach the goal of < 40 mg/dl (1 mmol/l) [2–5]. The SANTORINI study, which was performed in 2020–2021, showed a small improvement of 2.7% over the DaVinci study which was completed in 2019 [6]. However, still only 20.7% of atherosclerotic cardiovascular disease (ASCVD) patients achieved their LDL-C goal. This study also clearly showed that these disappointing findings are associated with a very poor therapeutical approach in patients with cerebral ASCVD and peripheral artery disease (PAD) – with only 15 and 18.6% reaching LDL-C goals, respectively [6]. The latter group present a real unmet need in most of the departments of cardiology in Europe, as PAD is very rarely diagnosed – despite the recognition of a very strong link between PAD and ASCVD and cardiovascular events. Unfortunately, the majority of these patients are now diagnosed and treated by the interventional surgeons [7, 8]. The results of the REALITY cohort study, performed in 2019 based on the nationwide database with real-life Spanish patients, confirmed that only about 3% of individuals achieve the LDL-C target of less than 55 mg/dl (1.4 mmol/l) and less than 15% of ASCVD patients reach the goal of < 70 mg/dl (1.8 mmol/l) [9]. This study again confirmed that the largest unmet needs refer to patients with stroke/transient ischemic attack (TIA) and PAD, for whom the 24-month mortality rate was between 10.2–11.9% [9–11].
The main reason for such a large extent of underperformance is the lack of use of high intensity statin (HIS) therapy. Less than 25% patients received HIS therapy in the SANTORINI study [6]. The corresponding figure was 32% in CEE countries and 38% in the Europe based on the DaVinci study [2, 3]. Comparing this data with the results of the Hyperlipidaemia Therapy in tERtiary Cardiological cEnTer (TERCET) Registry for ACS patients, which includes data up to 2016, it appears that there has been no improvement, as 6 years ago we observed the HIS ratio of 37.4% and 39.1% in patients with STEMI and NSTEMI, respectively [12]. Even more dramatic examples of underuse of effective therapies are seen for patients treated with the combination therapy of statin and ezetimibe, and double/triple therapy with PCSK9 inhibitors [2–6, 13, 14]. In the TERCET registry the proportion of patients on the combination therapy with ezetimibe was only 0.3% of patients with myocardial infarction (MI), and 3.5% in those with stable coronary artery disease [12]. In the DaVinci study, 9% of ASCVD patients were treated with combination therapy, and in the SANTORINI study it was 17.5% (and only 8.5% and 12% in those with cerebral ASCVD and PAD, respectively) [2, 3, 6]. There are still very few patients (only 4.7%) receiving combination therapy with PCSK9 inhibitors [6] (in the DaVinci it was only 1% [1]). This represents an enormous under-use of therapy considering the needs of patients. Based on modelling studies, a PCSK9 targeted approach therapy is expected to be administered in 20–22% of patients to achieve LDL-C goals. This means that presently, only one fifth of patients benefits from these innovative therapies) [15, 16].
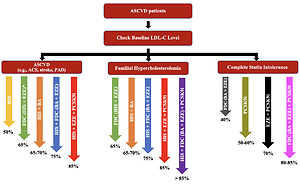
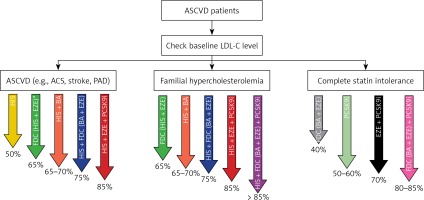
This relatively small improvement in the number of patients being treated with LLT combination therapy has not been accompanied by an improvement in the proportion of patients achieving LDL-C goals [17]. There are several reasons for this. The first is obviously associated with too few patients being treated with combination therapy – and in particular, insufficient use of upfront combination LLT. This is despite our International Lipid Expert Panel (ILEP) recommendations on upfront combination therapy, which were published in April 2021 [14], and the reiteration of the advice in the European Atherosclerosis Society (EAS) Task Force paper [18] as well as an expert opinion paper published in EHJ [19], and at least several national guidelines in which this approach was approved (e.g., in Poland, Spain, France) [7, 20–22]. Another reason for limited goal achievement is related to the fact that when non-statin drug(s) are added to statin therapy, the statin dose is reduced at the same time in many patients, and/or ezetimibe is discontinued in case of triple therapy with a PCSK9 targeted therapy approach. This was clearly demonstrated in the GOULD Registry, where after 2 years only 17.1% patients had LLT intensification, and in the PCSK9 inhibitors group there was LLT de-escalation in 18.6% of patients, and statin downtitration/discontinuation and ezetimibe discontinuation in 10.2% [23]. The same trend was observed in an analysis of 1499 patients included to the Managed Care for Acute Myocardial Infarction Survivors (MACAMIS; “KOS-Zawał”) program in Poland, where after 12 months it was observed that LLT was deescalated in 23.5% and intensified in only 9.2% of patients after ACS [24]. That is why all forthcoming recommendations should focus not only on when and how to intensity LLT, but to focus on intensification of each step (preferably with the use of FDC with the highest tolerated statin doses), and without discontinuation of the ezetimibe, which has become an unfortunate trend for many physicians treating patients with a PCSK9 targeted therapy approach. The results of the ODYSSEY APPRISE (and many other available reports) clearly showed that in patients treated with alirocumab, keeping patients on high intensity statin therapy and ezetimibe was associated with significantly larger number of patients reaching LDL-C goals (by as much as 3.5% and 13.4%, respectively) [5]. Therefore, to treat our patients effectively, we should always retain high-intensity statin therapy while adding ezetimbe, and HIS and ezetimbe while adding PCSK9 inhibitors. This approach would lead to an expected 80–85% reduction of LDL-C in comparison to the 65-70% seen in most current real-life observations [25, 26] (Figure 1).
Figure 1
How to be effective with lipid lowering therapy in ASCVD patients (the size of the LDL-C reduction for some recommended combinations is an assumption and still needs to be confirmed)
*FDC of high intensity statin therapy and ezetimibe is a preferable option for all ASCVD patients. ASCVD – atherosclerotic cardiovascular disease, LDL-C – low-density lipoprotein cholesterol, ASC – acute coronary syndrome, PAD – peripheral artery disease, HIS – high intensity statin therapy, FDC – fixed dose combination, EZE – ezetimibe, BA – bempedoic acid, PCSK9i – proprotein convertase subtilisin/kexin 9 targeted approach therapy (PCSK9 inhibitors + inclisiran).
Obviously, downtitration is possible in the case of statin-associated muscle symptoms (SAMS)/statin intolerance (SI). But in most cohort- and observational studies SI is overdiagnosed – which report prevalence to be as high as 30–40%. In the TERCET registry, worryingly, 9.3% of ACS patients received no statin therapy (and a much higher proportion received only low-to moderate intensity statin therapy) [12]. In the DaVinci study 6% received no statin, and additionally 46% only received low to moderate doses of statins in monotherapy) [2]. In the SANTORINI study, as many as 21.4% of ASCVD patients received no statin [6]. It is worth emphasizing that the true prevalence of SI is only < 7% when it is diagnosed using approved definitions [27]. After the exclusion of the drucebo effect [28, 29] (using an approach which considers patient education [28], by exclusion of conditions and risk factors that might increase of the SI risk and the use of the SAMS-Clinical Index Score), complete statin intolerance is observed in only about 2% of treated patients [30, 31]. Therefore, to provide effective treatment, it is not enough to use effective drugs at high doses, we must also carefully and effectively manage SI [32].
Finally, we also need to have clear and unanimous guidelines. Unfortunately, the European Society of Cardiology (ESC) prevention guidelines published in September 2021 recommended a stepwise approach to achieve LDL-C goal for ASCVD patients [33]. Meanwhile, evidence-based medicine (EBM) supports the paradigm of “the lower the better for longer” and “the earlier on LDL-C goal, the better” [34], which therefore requires intensive treatment as early as possible. The stepwise guidelines promote an approach, which was applied in most departments of cardiology about 15–20 years ago – and which is a main reason of very small ratio of ASCVD patients reaching LDL-C goals (irrespectively what goal was based on the applicable guidelines). The consequence of missed goals is the high number of recurrent CVD events and mortality (which may be as high as 20% in the first 12 months after ACS) [35, 36]. The reevaluation of the risk, looking again at comorbidities and considering patients’ preferences in those at the very high and extremely high CVD risk (e.g. in those after stroke or ACS), were, based on the authors’ guidelines, intended to improve the effectiveness of therapy [33]. However, the guidelines introduced considerable confusion in practice because they promoted the opposite approach to the ESC/EAS lipid guidelines published in 2019 [37], and contrasted with the upfront combination therapy approach, which has been widely used in last 2 years [28]. This may be the reason that fewer and fewer patients will achieve LDL-C goals (e.g., those who, based on these guidelines express a preference against further LLT intensification). Alternatively, the goal be met, but achievement of the target will be delayed (against the rule – ‘the earlier the better’).
Taking this opportunity, it is also worth at least briefly discussing at least 3 issues, that are raised by supporters of the stepwise approach:
Cost-effectiveness. It is said that the stepwise approach is more cost-effective. However, this claim cannot be substantiated because the approach essentially prolongs the time to achievement of the LDL-C target, as well as reduces the number of patients achieving the goal at all (see below). Abundant data indicates the direct link between unachieved therapeutic goals, and increased risk of CVD events (and therefore long-term costs). This does not even require a health technology assessment (HTA) analysis. An approach allowing earlier achievement and better maintenance of the LDL-C goals (assuming better adherence with the fixed doses combination (FDC) therapy, and earlier introduction of PCSK9 targeted approach) is the best way to reduce the risk of recurrent CVD events (and their consequences – costs of the interventional procedures, costs of heart failure therapy, sick leave/disability, and rehabilitation) and mortality [38, 39].
Potential risk in the elderly and in frailty patients (over 75 years of age). We now have convincing data suggesting that intensive LLT therapy in elderly patients with ASCVD (especially after an event) is as beneficial in this group as it is for younger groups [7, 40]. Additionally, an approach involving the administration of the upfront LLT with intensive statin therapy (but not necessarily at the highest-possible doses – atorvastatin 40 mg and rosuvastatin 20 mg) with ezetimbe as FDC may not only decrease the risk of possible SAMS (in comparison to HIS with the highest doses) [27] but may also increase adherence and the number of patients reaching LDL-C targets. Clinical practice clearly shows us that in most of these patients, statin therapy and combination therapy with statin (even in moderate intensity doses) and ezetimibe is sufficient for most of these patients to reach LDL-C goals. This is probably due to physiologically lower baseline LDL-C levels in comparison to younger groups [7, 37]. Obviously individualization of the therapy for this group of patients is highly recommended.
Lack of data confirming that the upfront lipid lowering therapy is effective, especially in the context of the CVD events reduction. Since the introduction of ezetimibe in 2002 (and in Poland since 2006) we have had numerous data suggesting that combination therapy (also as FDC) is significantly more effective in comparison to maximally tolerated statin therapy and/or to double the dose of statin [7, 12]. Additionally, high intensity statin therapy is associated with approximately 50% reduction of LDL-C, whereas for the combination therapy the expected reduction is as high as 65% [7, 37]. In a systematic review and meta-analysis of 13 studies with 5080 patients, the authors showed a significantly greater percentage reduction in LDL-C levels in patients treated with ezetimibe-statin vs. statin monotherapy (by –14.1%, p < 0.001) [41]. Reduction of LDL-C levels attributed to add-on ezetimibe was significantly greater than that for statin dose doubling (by –15.3%, p < 0.001), and LDL-C goal achievement favoured add-on ezetimibe over statin titration by as much as 2.5 times (p = 0.007) [41]. In a pooled analysis of 27 clinical trials with 21,000 subjects therapy with ezetimibe plus statin produced significantly greater reductions in LDL-C, total-cholesterol, non-high density lipoprotein cholesterol (HDL-C), apolipoprotein (Apo)B, triglycerides, and significantly higher achievement of LDL-C < 70 mg/dl by 18.2%, and < 100 mg/dl by 23.4% (p < 0.0001 for all) [42]. The same results were observed for both coronary heart disease patients (LDL-C < 70 mg/dl was achieved more frequently in statin/ezetimbe group by 21.8%), and in diabetic patients (< 100 mg/dl by 20.2%) [42]. Katzmann et al. studied prescription trends in oral non-statin LLT and their effects on LDL-C in Germany [43]. Data from 311,242 patients were analysed. They confirmed that addition of ezetimibe in patients already prescribed a statin reduced LDL-C by an additional 23.8% (32.3 ±38.4 mg/dl), with a greater reduction with FDC (reduction 28.4% (40.0 ±39.1 mg/dl)) as compared to separate pills (19.4% (27.5 ±33.8 mg/dl)); p < 0.0001. Despite a small proportion of patients reaching the recommended LDL-C level of < 70 mg/dl, a greater proportion of patients treated with FDC reached the goal (31.5%) compared to those with separate pills (21.0%) [43]. In a randomised, open-label, non-inferiority trial, 3780 ASCVD patients were randomly assigned to receive either moderate-intensity statin with ezetimibe combination therapy (rosuvastatin 10 mg with ezetimibe 10 mg; n = 1894) or high-intensity statin monotherapy (rosuvastatin 20 mg; n = 1886) [44]. The primary endpoint was the 3-year composite of cardiovascular death, major cardiovascular events, or non-fatal stroke. The primary endpoint occurred in 172 (9.1%) patients in the combination therapy group and 186 (9.9%) patients in the high-intensity statin monotherapy group (absolute difference –0.78%; 95% CI: –2.39–0.83). LDL-C concentrations of < 70 mg/dl at 1, 2, and 3 years were observed in 73%, 75%, and 72% of patients in the combination therapy group, and 55%, 60%, and 58% of patients in the high-intensity statin monotherapy group (all p < 0.0001). The combination therapy was also significantly better tolerated – discontinuation or dose reduction of the study drug by intolerance was observed in 88 (4.8%) patients and 150 (8.2%) patients, respectively (p < 0.0001) [44]. Finally, based on data from 38,023 consecutive patients with ACS from the Polish Registry of Acute Coronary Syndromes (PL-ACS) we showed that there was a significant difference between statin monotherapy (atorvastatin or rosuvastatin) and upfront combination therapy of statin and ezetimibe after 1 year (5.9 vs. 3.6%, p = 0.049), 2 (7.8% vs. 4.4%, p = 0.023) and 3 years (10.2% vs. 5.6%, p = 0.03) of follow-up (as well as for the overall period) – in favor for the upfront combination therapy [45]. Upfront combination therapy was associated with a significant reduction of all-cause mortality in comparison to statin monotherapy (OR = 0.490, 95% CI: 0.351–0.683) with absolute risk reduction (ARR) of 4.6% after 3 years (number needed to treat – NNT = 22) [45].
In conclusion, in recent decades we have made many mistakes in the management of lipid disorders, from completely ignoring this very common risk factor, to incorrect risk stratification, late diagnosis, and ineffective treatment with low doses and without the use of combination therapy [46–49]. In 2022, we have so many effective drugs (including new agents close on the horizon), and effective and scientifically confirmed approaches to treatment. Therefore, we simply cannot afford to lose our patients due to our own ineffectiveness and therapeutic inertia [50]. So, starting from now #ThinkUpfront!
Conflict of interest
Maciej Banach: speakers bureau: Amgen, Daichii Sankyo, KRKA, Polpharma, Mylan/Viatris, Novartis, Novo-Nordisk, Sanofi-Aventis, Teva, Zentiva; consultant to Amgen, Daichii Sankyo, Esperion, NewAmsterdam, Novartis, Novo-Nordisk, Polfarmex, Sanofi-Aventis; Grants from Amgen, Daichii Sankyo, Mylan/Viatris, Sanofi and Valeant; Zeljko Reiner: speakers bureau: Sanofi, Novartis; Margus Viigimaa: speakers bureau: Amgen, Novartis, Novo-Nordisk, Sanofi-Aventis, Menarini; Arman Postadzhiyan: speakers bureau: Amgen, Novartis, Novo-Nordisk, Teva, Zentiva; Daniel Pella: received honoraria for events sponsored by Amgen, Jamieson, Novartis, MSD, Pfizer, Servier; Peter E. Penson owns four shares in AstraZeneca PLC and has received honoraria and/or travel reimbursement for events sponsored by AKCEA, Amgen, AMRYT, Link Medical, Mylan, Napp, Sanofi; all other authors have no conflict of interest.