Current issue
Archive
Manuscripts accepted
About the Journal
Editorial office
Editorial board
Section Editors
Abstracting and indexing
Subscription
Contact
Ethical standards and procedures
Most read articles
Instructions for authors
Article Processing Charge (APC)
Regulations of paying article processing charge (APC)
NEUROLOGY / EXPERIMENTAL RESEARCH
β-estradiol alleviates hypoxic-ischemic brain damage in neonatal rats through the GPER1-mediated AKT/NF-κB signaling pathway
1
Department of Pediatrics, The First Affiliated Hospital of Harbin Medical University, Harbin, China
2
Department of Pediatrics, Linyi Maternal and Child Health Hospital, Linyi, China
3
Department of Genetic Laboratory, Linyi Maternal and Child Health Hospital, Linyi, China
4
Department of Key Laboratory of Birth Defects Prevention and Control, Linyi Maternal and Child Health Hospital, Linyi, China
5
Department of Neonatal, Linyi Maternal and Child Health Hospital, Linyi, China
6
Department of Reproductive Medicine, Linyi Maternal and Child Health Hospital, Linyi, China
Submission date: 2024-06-19
Final revision date: 2024-07-27
Acceptance date: 2024-08-05
Online publication date: 2024-08-06
Corresponding author
Xiangping Xu
Department of Pediatrics, The First Affiliated Hospital of Harbin Medical University, Harbin, 150001, China
Department of Pediatrics, The First Affiliated Hospital of Harbin Medical University, Harbin, 150001, China
KEYWORDS
hypoxic-ischemic brain damageoxygen-glucose deprivationestradiol 2G protein-coupled estrogen receptor 1GPER1 inhibitor (G15)AKTnuclear factor-κB
TOPICS
ABSTRACT
Introduction:
Research has established that estradiol (E2) offers neuroprotection against hypoxic-ischemic brain damage (HIBD) in neonatal rats, yet the underlying mechanisms are not fully understood. This study sought to determine whether E2’s neuroprotective effects in neonatal HIBD are mediated through astrocytes by modulating G protein-coupled estrogen receptor 1 (GPER1) and the subsequent AKT serine (AKT)/nuclear factor-κB (NF-κB) signaling cascade.
Material and methods:
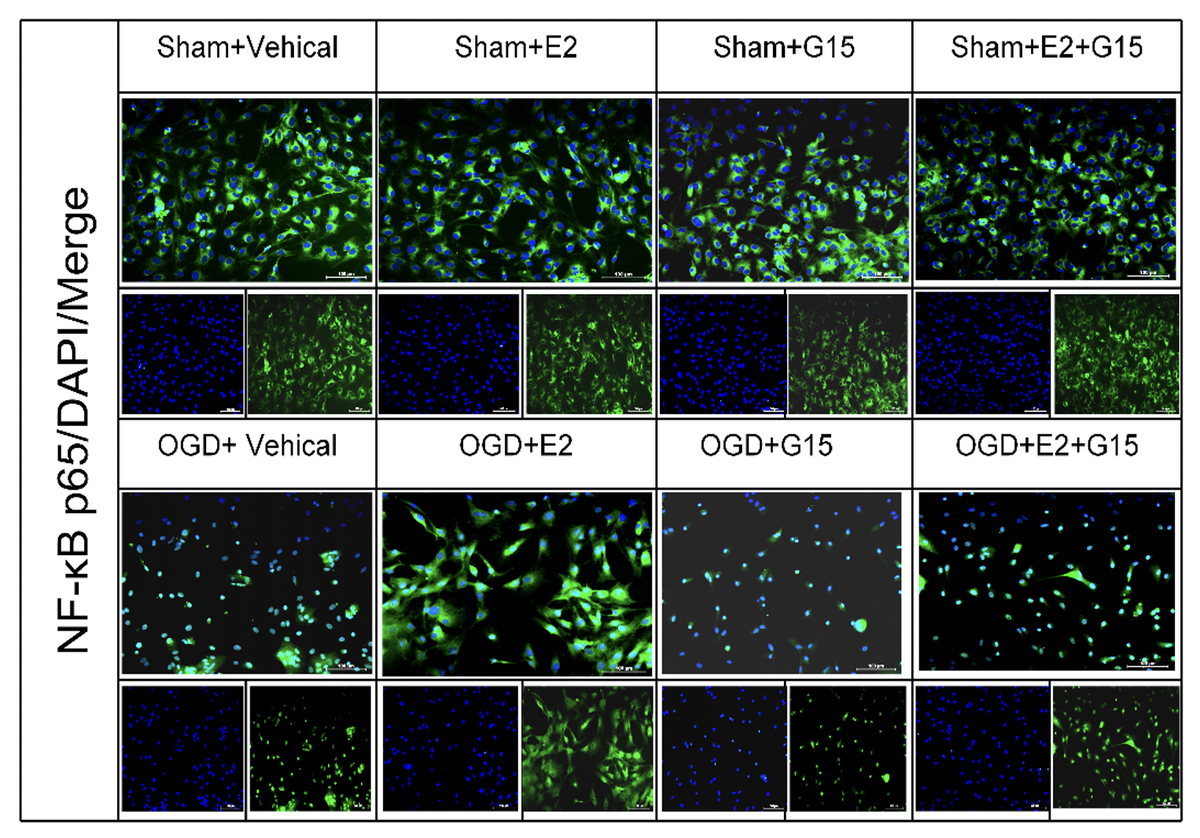
We developed an in vivo HIBD model in neonatal rats and established primary cultures of astrocytes subjected to oxygen-glucose deprivation-reoxygenation (OGD-R) as an in vitro model. E2 and the GPER1 inhibitor (G15) were administered according to the experimental design. Protein expression levels of GPER1, phosphorylated AKT (p-AKT), NF-κB p65, and cleaved caspase-3 were examined using Western blot analysis. Apoptosis was assessed via the TUNEL assay, and the presence of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in the cell supernatant was quantified by ELISA. The localization of p-AKT and NF-κB p65 was determined through immunofluorescence.
Results:
Our findings indicate that E2 treatment significantly reduced the volume of brain infarction and astrocyte apoptosis. E2 upregulated GPER1 and p-AKT expression while downregulating NF-κB p65 and cleaved caspase3 levels in astrocytes and neonatal rats after HIBD. Additionally, E2 diminished the secretion of TNF-α and IL-1β in the cell supernatant. The G15 inhibitor notably reversed the neuroprotective effects of E2 and the associated molecular changes.
Conclusions:
These results suggest that E2 may provide neuroprotection in neonatal rats with HIBD by inhibiting astrocyte apoptosis and modulating the expression of GPER1, p-AKT, and NF-κB, thereby providing a potential therapeutic strategy for HIBD.
Research has established that estradiol (E2) offers neuroprotection against hypoxic-ischemic brain damage (HIBD) in neonatal rats, yet the underlying mechanisms are not fully understood. This study sought to determine whether E2’s neuroprotective effects in neonatal HIBD are mediated through astrocytes by modulating G protein-coupled estrogen receptor 1 (GPER1) and the subsequent AKT serine (AKT)/nuclear factor-κB (NF-κB) signaling cascade.
Material and methods:
We developed an in vivo HIBD model in neonatal rats and established primary cultures of astrocytes subjected to oxygen-glucose deprivation-reoxygenation (OGD-R) as an in vitro model. E2 and the GPER1 inhibitor (G15) were administered according to the experimental design. Protein expression levels of GPER1, phosphorylated AKT (p-AKT), NF-κB p65, and cleaved caspase-3 were examined using Western blot analysis. Apoptosis was assessed via the TUNEL assay, and the presence of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in the cell supernatant was quantified by ELISA. The localization of p-AKT and NF-κB p65 was determined through immunofluorescence.
Results:
Our findings indicate that E2 treatment significantly reduced the volume of brain infarction and astrocyte apoptosis. E2 upregulated GPER1 and p-AKT expression while downregulating NF-κB p65 and cleaved caspase3 levels in astrocytes and neonatal rats after HIBD. Additionally, E2 diminished the secretion of TNF-α and IL-1β in the cell supernatant. The G15 inhibitor notably reversed the neuroprotective effects of E2 and the associated molecular changes.
Conclusions:
These results suggest that E2 may provide neuroprotection in neonatal rats with HIBD by inhibiting astrocyte apoptosis and modulating the expression of GPER1, p-AKT, and NF-κB, thereby providing a potential therapeutic strategy for HIBD.
REFERENCES (51)
1.
She HQ, Sun YF, Chen L, et al. Current analysis of hypoxic-ischemic encephalopathy research issues and future treatment modalities. Front Neurosci 2023; 17: 1136500.
2.
Xu K, Zhang LF, Zhang L, et al. MiR-125b-5p enclosed in hypoxic HK2 cell-derived extracellular vesicles alleviates renal ischemia-reperfusion injury by regulating NLRC5. Arch Med Sci 2024. DOI: https://doi.org/10.5114/aoms/1....
3.
Silveira RC, Procianoy RS. Hypothermia therapy for newborns with hypoxic ischemic encephalopathy. J Pediatr (Rio J) 2015; 91: S78-83.
4.
Rao R, Trivedi S, Vesoulis Z, et al. Safety and short-term outcomes of therapeutic hypothermia in preterm neonates 34-35 weeks gestational age with hypoxic-ischemic encephalopathy. J Pediatr 2017; 183: 37-42.
5.
Wang Z, Zhang D, Zhang P, et al. Safety and efficacy of therapeutic hypothermia in neonates with mild hypoxic-ischemic encephalopathy. BMC Pediatr 2023; 23: 530.
6.
Brown C, Suzuki S, Jelks K, Wise P. Estradiol is a potent protective, restorative, and trophic factor after brain injury. Semin Reprod Med 2009; 27: 240-9.
7.
Liu YB, Gao XT, Huang LY, Liu XL. Clinicopathological characteristics and prognostic factors in invasive micropapillary carcinoma of the breast. Arch Med Sci 2024; 20: 428-35.
8.
Markiewicz I, Lukomska B. The role of astrocytes in the physiology and pathology of the central nervous system. Acta Neurobiol Exp (Warsz) 2006; 66: 343-58.
9.
Kunc M, Gabrych A, Dulak D, et al. Systemic inflammatory markers and serum lactate dehydrogenase predict survival in patients with Wilms tumour. Arch Med Sci 2022; 18: 1253-61.
10.
Swanson R, Ying W, Kauppinen T. Astrocyte influences on ischemic neuronal death. Curr Mol Med 2004; 4: 193-205.
11.
Wilson JX. Antioxidant defense of the brain: a role for astrocytes. Can J Physiol Pharmacol 1997; 75: 1149-63.
12.
Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci 2007; 10: 1377-86.
13.
Xu L, Emery JF, Ouyang Y, Voloboueva LA, Giffard RG. Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia 2010; 58: 1042-9.
14.
Bessa A, Campos FL, Videira RA, et al. GPER: a new tool to protect dopaminergic neurons? Biochim Biophys Acta 2015; 1852: 2035-41.
15.
Wang J, Yu R, Han QQ, et al. G-1 exhibit antidepressant effect, increase of hippocampal ERs expression and improve hippocampal redox status in aged female rats. Behav Brain Res 2019; 359: 845-52.
16.
Zhang Z, Qin P, deng Y, et al. The novel estrogenic receptor GPR30 alleviates ischemic injury by inhibiting TLR4-mediated microglial inflammation. J. Neuroinflammation 2018; 15: 206.
17.
Karki P, Smith K, Johnson J, Lee E. Astrocyte-derived growth factors and estrogen neuroprotection: role of transforming growth factor- in estrogen-induced upregulation of glutamate transporters in astrocytes. Mol Cell Endocrinol 2014; 389: 58-64.
18.
Peso LD, González-Garcıa M, Page C, Herrera R, Nuñez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 1997; 278: 687-9.
19.
Tang H, Zhang Q, Yang L, et al. Reprint of ”GPR30 mediates estrogen rapid signaling and neuroprotection”. Mol Cell Endocrinol 2014; 389: 92-8.
20.
Jones WK, Brown M, Ren X, He S, McGuinness M. NF-B as an integrator of diverse signaling pathways the heart of myocardial signaling? Cardiovasc Toxicol 2003; 3: 229-54.
21.
Ivanova T, Mendez P, Garcia-Segura LM, Beyer C. Rapid stimulation of the PI3-kinase/Akt signalling pathway in developing midbrain neurones by oestrogen. J. Neuroendocrinol 2002; 14: 73-9.
22.
Jover-Mengual T, Miyawaki T, Latuszek A, et al. Acute estradiol protects CA1 neurons from ischemia-induced apoptotic cell death via the PI3K/Akt pathway. Brain Res 2010; 1321: 1-12.
23.
Yang L, Lu L, Yue J, et al. Activation of microglial G protein-coupled receptor 30 protects neurons against excitotoxicity through NF-B/MAPK pathways. Brain Res Bull 2021; 172: 22-30.
24.
Xu N, Zhang Y, Doycheva DM, et al. Adiponectin attenuates neuronal apoptosis induced by hypoxia-ischemia via the activation of AdipoR1/APPL1/LKB1/AMPK pathway in neonatal rats. Neuropharmacology 2018; 133: 415-28.
25.
Rice JE, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 1981; 9: 131-41.
26.
Gamdzyk M, Doycheva DM, Araujo C, et al. cGAS/STING pathway activation contributes to delayed neurodegeneration in neonatal hypoxia-ischemia rat model: possible involvement of LINE-1. Mol Neurobiol 2020; 57: 2600-19.
27.
McCarthy KD, De Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 1980; 85: 890-902.
28.
Giovannoni F, Quintana FJ. The role of astrocytes in CNS inflammation. Trends Immunol 2020; 41: 805-19.
29.
Kaur C, Ling E. Blood brain barrier in hypoxic-ischemic conditions. Curr Neurovasc Res 2008; 5: 71-81.
30.
Nawashiro H, Brenner M, Fukui S, Shima K, Hallenbeck JM. High susceptibility to cerebral ischemia in GFAP-null mice. J Cereb Blood Flow Metab 2000; 20: 1040-4.
31.
Kinoshita A, Yamada K, Kohmura E, Hayakawa T. Effect of astrocyte-derived factors on ischemic brain edema induced by rat MCA occlusion. APMIS 1990; 98: 851-7.
32.
Nuñez J, Yang Z, jiang Y, et al. 17-Estradiol protects the neonatal brain from hypoxia–ischemia. Exp Neurol 2007; 208: 269-76.
33.
Gerstner B, lee J, DeSilva TM, et al. 17-estradiol protects against hypoxic/ischemic white matter damage in the neonatal rat brain. J Neurosci Res 2009; 87: 2078-86.
34.
Kumar RS, Goyal N. Estrogens as regulator of hematopoietic stem cell, immune cells and bone biology. Life Sci 2021; 269: 119091.
35.
Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol 2019; 116: 135-70.
36.
Lu Y, Sareddy GR, Wang J, et al. Neuron-derived estrogen is critical for astrocyte activation and neuroprotection of the ischemic brain. J Neurosci 2020; 40: 7355-74.
37.
Ma Y, Guo H, Zhang L, et al. OPEN Estrogen replacement therapy-induced neuroprotection against brain ischemia-reperfusion injury involves the activation of astrocytes via estrogen receptor. Sci Rep 2016; 6: 21467.
38.
Wise PM, Suzuki S, Brown CM. Estradiol: a hormone with diverse and contradictory neuroprotective actions. Dialogues Clin Neurosci 2009; 11: 297-303.
39.
DeLeon C, Pemberton K, Green M, et al. Novel GPER agonist, CITFA, increases neurite growth in rat embryonic (E18) hippocampal neurons. ACS Chem Neurosci 2022; 13: 1119-28.
40.
Zhao TZ, Shi F, Hu J, et al. GPER1 mediates estrogen-induced neuroprotection against oxygen-glucose deprivation in the primary hippocampal neurons. Neuroscience 2016; 328: 117-26.
41.
Wang XS, Yue J, Hu LN, et al. Activation of G protein-coupled receptor 30 protects neurons by regulating autophagy in astrocytes. Glia 2020; 68: 27-43.
42.
Ariyani W, Miyazaki W, Koibuchi N. A novel mechanism of S-equol action in neurons and astrocytes: the possible involvement of GPR30/GPER1. Int J Mol Sci 2019; 20: 5178.
43.
Sandén C, Broselid S, Cornmark L, et al. G protein-coupled estrogen receptor 1/G protein-coupled receptor 30 localizes in the plasma membrane and traffics intracellularly on cytokeratin intermediate filaments. Mol Pharmacol 2011; 79: 400-10.
44.
Ruiz-Palmero I, Hernando M, Garcia-Segura LM, Arevalo MA. G protein-coupled estrogen receptor is required for the neuritogenic mechanism of 17-estradiol in developing hippocampal neurons. Mol Cell Endocrinol 2013; 372: 105-15.
45.
Chen J, Hu R, Ge H, et al. G-protein-coupled receptor 30-mediated antiapoptotic effect of estrogen on spinal motor neurons following injury and its underlying mechanisms. Mol Med Rep 2015; 12: 1733-40.
46.
Ozcan L, Otunctemur A, Polat EC, et al. Selective nuclear factor kappa b (NFkB) inhibitor, pyrrolidium dithiocarbamate prevents, long-term histologic damage in ischemia-reperfusion injuries after delayed testicular torsion. Urol J 2016; 13: 2702-6.
47.
Oliveira-Marques V, Marinho HS, Cyrne L, Antunes F. Role of hydrogen peroxide in NF-B activation: from inducer to modulator. Antioxid Redox Signal 2009; 11: 2223-43.
48.
Song YS, Narasimhan P, Kim GS, et al. The role of Akt signaling in oxidative stress mediates NF-kB activation in mild transient focal cerebral ischemia. J Cereb Blood Flow Metab 2008; 28: 1917-26.
49.
Guha M, Mackman N. The phosphatidylinositol 3-kinase-akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem 2002; 277: 32124-32.
50.
Li L, Mcbride DW, Doycheva D, et al. G-CSF attenuates neuroinflammation and stabilizes the blood–brain barrier via the PI3K/Akt/GSK-3 signaling pathway following neonatal hypoxia-ischemia in rats. Exp Neurol 2015; 272: 135-44.
51.
Simpkins JW, Singh M, Brock C, Etgen AM. Neuroprotection and estrogen receptors. Neuroendocrinology 2012; 96: 119-30.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.